Introduction
Epstein-Barr (EBV) viraemia is frequently encountered in the context of haemophagocytic lymphohistiocytosis (HLH), systemic inflammation and lymphoproliferative disease (LPD). Although EBV typically infects B lymphocytes triggering both polyclonal proliferation and lymphoma, EBV can also infect T and NK cells. Understanding the phenotype of EBV-infected cells can help identify the underlying diagnosis and guide the use of appropriate targeted therapy. We have developed an EBV FlowRNA assay combining the detection of EBV-encoded small RNA (EBER) with multicolour flow cytometry (Collins, Blood, 2020). Here we present a larger case series illustrating how this assay can be used in diagnostic workup.
Methods
Anticoagulated blood samples were received from patients with EBV viraemia and either HLH or confirmed or suspected LPD within 36 hours of collection. Mononuclear cells were isolated by density centrifugation and one to three million cells were stained with a fixable viability marker and conjugated antibodies to cell surface markers for lymphocyte immunophenotyping. FlowRNA staining was then performed as per manufacturers' instructions (PrimeFlow RNA, Thermo Fisher), using target probe sets (Thermo Fisher) for EBER 1/2 and Beta-2-microglobulin (B2M). The sample was then acquired on a LSRFortessa X-20 cytometer and subsequent analysis performed in FlowJo version 10.
Results
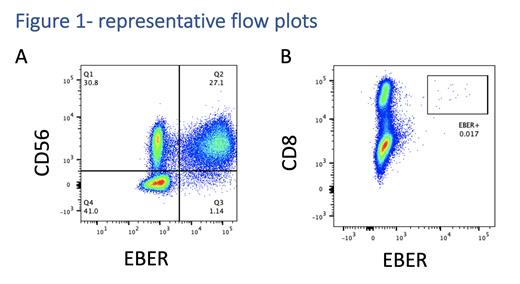
Between February 2021 and June 2023, we received 23 adequate samples from 19 patients. A definitive EBV+ population was identified in single lymphocyte subsets from 15 samples. Significant EBV-infected subsets were identified; CD19+ B cells from 4 patients, CD56+ NK cells from 6 patients, CD4+ T cells from 1 patient and CD8+ T cells from 4 patients. All patients with EBV-infected T/NK cells had an underlying lymphoma. The frequency of infected cells detected ranged from 0.017% in a case of nodal CD8+ T cell lymphoma (figure A) to 22% in a patient with relapsed extra-nodal NK T lymphoma (ENKTL) with heavy marrow infiltration and an EBV viral load of 1x10 6 copies per ml (figure B). In 5 samples no EBER+ cells were identified despite EBV DNAemia of up 2x10 6 copies per ml. Although a small number of EBER+ events could be detected in a further two samples, the very low frequency of EBV+ cells made it impossible to confirm this was a genuine population.
Discussion
FlowRNA can be used to give useful clinical information with a short turnaround time. In two patients with suspected relapse of ENKTL, CD56+EBER+ cells could be detected in the peripheral blood despite no lesions being visible with imaging. The diagnosis was only later confirmed by bone marrow biopsy. Importantly, FlowRNA enables the distinction between EBV positivity in circulating tumour cells from bystander B cell reactivation. In one patient with known T cell lymphoma and EBV DNAemia, the B cells were EBER+ but tumour cells were negative confirming bystander infection. Conversely, in another patient with CD8+ nodal lymphoma, EBER+ T cells were identifiable pre and post-treatment. This technique can therefore be used to monitor therapy and has the potential to identify measurable residual disease (MRD). Clonality of EBER+ B cells can be confirmed by Kappa/Lambda staining; in two cases of EBV-related HLH, EBER detection in polyclonal B lymphocytes helped confirm EBV-related lymphoproliferation rather than underlying lymphoma.
The assay can be highly sensitive, detecting very small lymphocyte populations. However, to achieve this at least 100,000 lymphocytes must be analysed. Measuring sufficient cell numbers can be challenging in the context of post-treatment lymphopenia, although using up to 50 ml of blood can increase the cell yield. Additionally, this technique can only detect EBER+ cells when present in the peripheral blood. We have noted negative FlowRNA with high EBV viral load in conditions previously associated with cell-free viral DNA including ENKTL and Hodgkin lymphoma, which represented 3/5 negative samples.
FlowRNA has several significant strengths, it is highly sensitive, reproducible, has a short turnaround time and by its nature as a blood test, samples can be easily obtained. The ability to monitor low-frequency tumour cells in the blood gives potential for MRD monitoring. We are now working to validate this test for routine diagnostic use.
Disclosures
Glover:Jansen: Research Funding. Ahearne:Pfizer: Research Funding; Takeda: Other: Conference travel support. Collins:Amgen: Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; Astra Zeneca: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Beigene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Honoraria; BMS: Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Fox:AbbVie: Consultancy; Genmab: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shannon-Lowe:Jansen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal